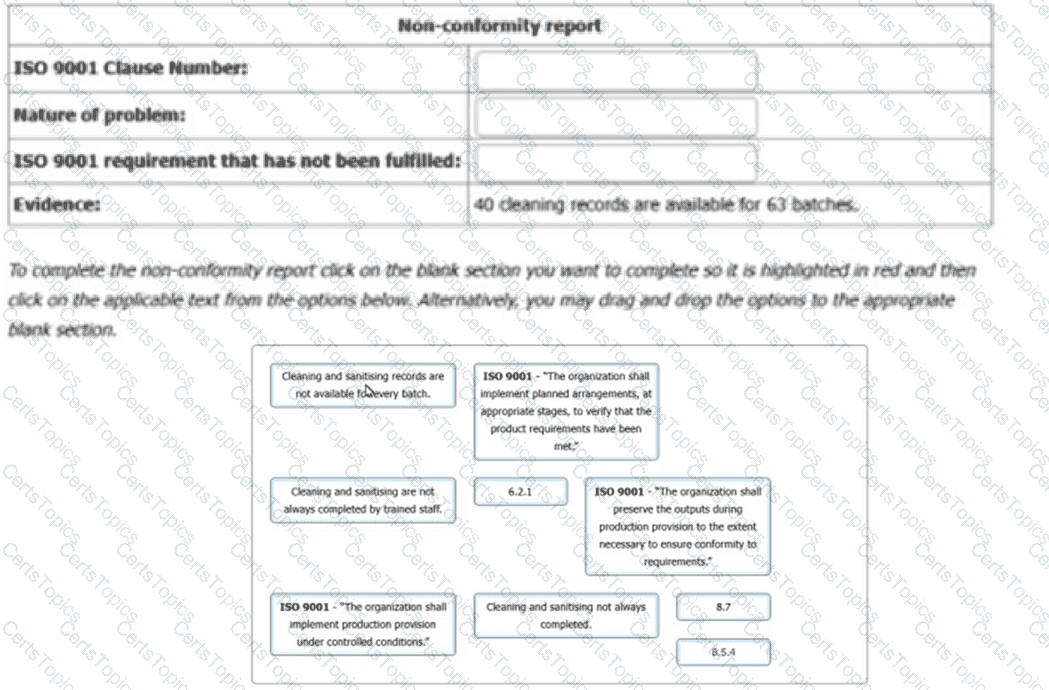
Which of the following is a record related to the audit program that should be managed and maintained?
You are carrying out an annual surveillance audit at an organisation that has been certificated to ISO 9001 for two years. The organisation offers home cleaning services. The scope of the quality management system covers planning the weekly activities, providing cleaning materials, cleaning the whole property (including outdoor space) alarm installation, alarm servicing, alarm monitoring and response. The business operates from a single office and employs subcontract cleaners across the whole city.
You have just completed the opening meeting. You are interviewing the Managing Director (MD).
You: I would like to gain an understanding of how the quality management system has been supporting your business and its strategic direction.
MD: We are continuing to face difficult times. The market is extremely competitive, and customers typically look for the least expensive option when choosing home cleaning services. We have not yet seen any business benefit from our quality management system.
You: Tell me how you determine external and internal issues.
MD: We use PESTLE analysis (Political, economic, social, technological, legal, environmental).
You: Why did you not use the SWOT model (Strengths, Weaknesses, Opportunities, Threats)?
MD: I had used PESTLE in my previous job.
You: How have the outputs from your PESTLE been used?
Select two audit trails which would lead to a determination of how the PESTLE analysis would affect the planning of a QMS to ISO 9001.
You are a member of the audit team of a second-party audit of an organisation with 625 employees. The audit procedure recommends using sampling criteria which requires the review of the documented competence for 25 personnel. The audit team leader developed an audit plan allocating one hour to audit the Human Resources department (from 11:30 am to 12:30 pm). She told you that she could not allocate any additional time.
What would you do?
You are carrying out an audit at a single-site organisation seeking certification to ISO 9001 for the first time. The organisation manufactures cosmetics for major retailers.
You are interviewing the Manufacturing Manager (MM).
You: "I would like to begin by looking at the cleaning controls."
MM: "We record the cleaning of the equipment at the end of every batch. This document details the minimum cleaning frequency and the procedures to follow for all areas and each item of equipment. The person who carries out the cleaning puts their initial on the document and records the time and date alongside."
Narrative: You sample production records over 3-days and note down evidence of nonconformity as per the table below.
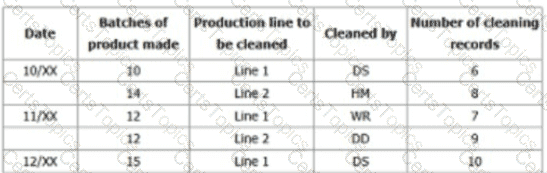
You decide to raise a non-conformity.