A polar bear’s furry coat protects the bear from the frigid air temperature. Similarly, a covering of ice floating on a pond of water protects the fish that inhabit the pond.
Why does water remain in a liquid state below the surface of ice?
Based on the diagram,

at which point is the least amount of force needed to move the weighted box?
In a population cycle, predators eat and reduce the number of prey. If the number of predators is reduced, the number of prey will gradually increase. Eventually, the predator population will increase, and the population cycle will continue.
Which pair best demonstrates a predator-prey relationship?
-- Exhibit --
The pH scale is a measure of the acidity of a substance. The numerical range of the scale is from 0.0 to 14.0. A midpoint pH of 7.0 designates a neutral substance (neither acid nor base). A pH lower than 7.0 denotes an acid substance, whereas a pH higher than 7.0 denotes a basic substance.
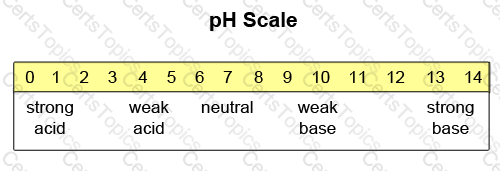
-- Exhibit --
Which of the following substances is the MOST basic?