The BEST roommate selection for a patient with active shingles would be a patient who has had
varicella vaccine.
treatment with acyclovir
a history of herpes simplex.
varicclla zoster immunoglobulin
A patient with active shingles (herpes zoster) is contagious to individuals who have never had varicella (chickenpox) or the varicella vaccine. The best roommate selection is someone who has received the varicella vaccine, as they are considered immune and not at risk for contracting the virus.
Why the Other Options Are Incorrect?
B. Treatment with acyclovir – Acyclovir treats herpes zoster but does not prevent transmission to others.
C. A history of herpes simplex – Prior herpes simplex virus (HSV) infection does not confer immunity to varicella-zoster virus (VZV).
D. Varicella zoster immunoglobulin (VZIG) – VZIG provides temporary immunity but does not offer long-term protection like the vaccine.
CBIC Infection Control Reference
APIC guidelines recommend placing patients with active shingles in a room with individuals immune to varicella, such as those vaccinated.
An infection preventionist (IP) is informed of a measles outbreak in a nearby community. What is the IP’s FIRST priority when working with Occupational Health?
Isolate employees who have recently traveled to areas with measles outbreaks.
Reassign employees who are pregnant from caring for patients with suspected measles.
Verify that employees in high-risk exposure areas of the facility have adequate immunity to measles.
Set up a mandatory vaccination clinic in collaboration with Occupational Health and local public health partners.
When an infection preventionist (IP) is informed of a measles outbreak in a nearby community, the immediate priority is to protect healthcare workers and patients from potential exposure, particularly in a healthcare setting where vulnerable populations are present. Working with Occupational Health, the IP must follow a structured approach to mitigate the risk of transmission, guided by principles from the Certification Board of Infection Control and Epidemiology (CBIC) and public health guidelines. Let’s evaluate each option to determine the first priority:
A. Isolate employees who have recently traveled to areas with measles outbreaks: Isolating employees who may have been exposed to measles during travel is an important infection control measure to prevent transmission within the facility. However, this action assumes that exposure has already occurred and requires identification of affected employees first. Without knowing the immunity status of the workforce, this step is reactive rather than preventive and cannot be the first priority.
B. Reassign employees who are pregnant from caring for patients with suspected measles: Reassigning pregnant employees is a protective measure due to the severe risks measles poses to fetuses (e.g., congenital rubella syndrome risks, though measles itself is more about maternal complications). This action is specific to a subset of employees and depends on identifying patients with suspected measles, which may not yet be confirmed. It is a secondary step that follows assessing overall immunity and exposure risks, making it inappropriate as the first priority.
C. Verify that employees in high-risk exposure areas of the facility have adequate immunity to measles: Verifying immunity is the foundational step in preventing measles transmission in a healthcare setting. Measles is highly contagious, and healthcare workers in high-risk areas (e.g., emergency departments, pediatric wards) are at increased risk of exposure. The CBIC and CDC recommend ensuring that all healthcare personnel have documented evidence of measles immunity (e.g., two doses of MMR vaccine, laboratory evidence of immunity, or prior infection) as a primary infection control strategy during outbreaks. This step allows the IP to identify vulnerable employees, implement targeted interventions, and comply with occupational health regulations. It is the most proactive and immediate priority when an outbreak is reported in the community.
D. Set up a mandatory vaccination clinic in collaboration with Occupational Health and local public health partners: Establishing a vaccination clinic is a critical long-term strategy to increase immunity and control the outbreak. However, this requires planning, resource allocation, and coordination, which take time. It is a subsequent step that follows verifying immunity status to identify those who need vaccination. While important, it cannot be the first priority due to its logistical demands.
The first priority is C, as verifying immunity among employees in high-risk areas establishes a baseline to prevent transmission before reactive measures (e.g., isolation, reassignment) or broader interventions (e.g., vaccination clinics) are implemented. This aligns with CBIC’s focus on proactive risk assessment and occupational health safety during infectious disease outbreaks, ensuring a rapid response to protect the healthcare workforce and patients.
References:
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain III: Prevention and Control of Infectious Diseases, which prioritizes immunity verification during outbreaks.
CBIC Examination Content Outline, Domain IV: Environment of Care, which includes ensuring employee immunity as part of outbreak preparedness.
CDC Guidelines for Measles Prevention (2023), which recommend verifying healthcare worker immunity as the initial step during a measles outbreak.
What inflammatory reaction may occur in the eye after cataract surgery due to a breach in disinfection and sterilization of intraocular surgical instruments?
Endophthalmitis
Bacterial conjunctivitis
Toxic Anterior Segment Syndrome
Toxic Posterior Segment Syndrome
The correct answer is C, "Toxic Anterior Segment Syndrome," as this is the inflammatory reaction that may occur in the eye after cataract surgery due to a breach in disinfection and sterilization of intraocular surgical instruments. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, Toxic Anterior Segment Syndrome (TASS) is a sterile, acute inflammatory reaction that can result from contaminants introduced during intraocular surgery, such as endotoxins, residues from improper cleaning, or chemical agents left on surgical instruments due to inadequate disinfection or sterilization processes (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). TASS typically presents within 12-48 hours post-surgery with symptoms like pain, redness, and anterior chamber inflammation, and it is distinct from infectious causes because it is not microbial in origin. A breach in reprocessing protocols, such as failure to remove detergents or improper sterilization, is a known risk factor, making it highly relevant to infection prevention efforts in surgical settings.
Option A (endophthalmitis) is an infectious inflammation of the internal eye structures, often caused by bacterial or fungal contamination, which can also result from poor sterilization but is distinguished from TASS by its infectious nature and longer onset (days to weeks). Option B (bacterial conjunctivitis) affects the conjunctiva and is typically a surface infection unrelated to intraocular surgery or sterilization breaches of surgical instruments. Option D (toxic posterior segment syndrome) is not a recognized clinical entity in the context of cataract surgery; inflammation in the posterior segment is more commonly associated with infectious endophthalmitis or other conditions, not specifically linked to reprocessing failures.
The focus on TASS aligns with CBIC’s emphasis on ensuring safe reprocessing to prevent adverse outcomes in surgical patients, highlighting the need for rigorous infection control measures (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). This is supported by CDC and American Academy of Ophthalmology guidelines, which identify TASS as a preventable complication linked to reprocessing errors (CDC Guidelines for Disinfection and Sterilization, 2019; AAO TASS Task Force Report, 2017).
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.3 - Ensure safe reprocessing of medical equipment, 3.5 - Evaluate the environment for infection risks. CDC Guidelines for Disinfection and Sterilization in Healthcare Facilities, 2019. AAO TASS Task Force Report, 2017.
The infection preventionist (IP) is working with the Product Evaluation Committee to select a sporicidal disinfectant for Clostridioides difficile. An effective disinfectant for the IP to recommend is
quaternary ammonium compound.
phenolic.
isopropyl alcohol.
sodium hypochlorite.
The correct answer is D, "sodium hypochlorite," as it is an effective sporicidal disinfectant for Clostridioides difficile that the infection preventionist (IP) should recommend. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, Clostridioides difficile (C. difficile) is a spore-forming bacterium responsible for significant healthcare-associated infections (HAIs), and its spores are highly resistant to many common disinfectants. Sodium hypochlorite (bleach) is recognized by the Centers for Disease Control and Prevention (CDC) and the Environmental Protection Agency (EPA) as a sporicidal agent capable of inactivating C. difficile spores when used at appropriate concentrations (e.g., 1:10 dilution of household bleach) and with the recommended contact time (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.4 - Implement environmental cleaning and disinfection protocols). This makes it a preferred choice for environmental disinfection in outbreak settings or areas with known C. difficile contamination.
Option A (quaternary ammonium compound) is effective against many bacteria and viruses but lacks sufficient sporicidal activity against C. difficile spores, rendering it inadequate for this purpose. Option B (phenolic) has broad-spectrum antimicrobial properties but is not reliably sporicidal and is less effective against C. difficile spores compared to sodium hypochlorite. Option C (isopropyl alcohol) is useful for disinfecting surfaces and killing some pathogens, but it is not sporicidal and evaporates quickly, making it ineffective against C. difficile spores.
The IP’s recommendation of sodium hypochlorite aligns with CBIC’s emphasis on selecting disinfectants based on their efficacy against specific pathogens and adherence to evidence-based guidelines (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). Proper use, including correct dilution and contact time, is critical to ensure effectiveness, and the IP should collaborate with the Product Evaluation Committee to ensure implementation aligns with safety and regulatory standards (CDC Guidelines for Environmental Infection Control in Healthcare Facilities, 2019).
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.4 - Implement environmental cleaning and disinfection protocols, 3.5 - Evaluate the environment for infection risks. CDC Guidelines for Environmental Infection Control in Healthcare Facilities, 2019.
Which of the following factors should be considered when evaluating countertop surface materials?
Durability
Sink design
Accessibility
Faucet placement
The correct answer is A, "Durability," as it is a critical factor to consider when evaluating countertop surface materials. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, the selection of materials in healthcare settings, including countertop surfaces, must prioritize infection prevention and control. Durability ensures that the surface can withstand frequent cleaning, disinfection, and physical wear without degrading, which is essential to maintain a hygienic environment and prevent the harboring of pathogens (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.4 - Implement environmental cleaning and disinfection protocols). Durable materials, such as solid surface composites or stainless steel, resist scratches, cracks, and moisture damage, reducing the risk of microbial growth and cross-contamination, which are significant concerns in healthcare facilities.
Option B (sink design) relates more to the plumbing and fixture layout rather than the inherent properties of the countertop material itself. While sink placement and design are important for workflow and hygiene, they are secondary to the material's characteristics. Option C (accessibility) is a consideration for user convenience and compliance with the Americans with Disabilities Act (ADA), but it pertains more to the installation and layout rather than the material's suitability for infection control. Option D (faucet placement) affects usability and water management but is not a direct attribute of the countertop material.
The emphasis on durability aligns with CBIC’s focus on creating environments that support effective cleaning and disinfection practices, which are vital for preventing healthcare-associated infections (HAIs). Selecting durable materials helps ensure long-term infection prevention efficacy, making it a primary factor in the evaluation process (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks).
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.4 - Implement environmental cleaning and disinfection protocols, 3.5 - Evaluate the environment for infection risks.
Which of the following stains is used to identify mycobacteria?
Acid-fast
Gram
Methylene blue
India ink
Mycobacteria, including species such as Mycobacterium tuberculosis and Mycobacterium leprae, are a group of bacteria known for their unique cell wall composition, which contains a high amount of lipid-rich mycolic acids. This characteristic makes them resistant to conventional staining methods and necessitates the use of specialized techniques for identification. The acid-fast stain is the standard method for identifying mycobacteria in clinical and laboratory settings. This staining technique, developed by Ziehl-Neelsen, involves the use of carbol fuchsin, which penetrates the lipid-rich cell wall of mycobacteria. After staining, the sample is treated with acid-alcohol, which decolorizes non-acid-fast organisms, while mycobacteria retain the red color due to their resistance to decolorization—hence the term "acid-fast." This property allows infection preventionists and microbiologists to distinguish mycobacteria from other bacteria under a microscope.
Option B, the Gram stain, is a common differential staining technique used to classify most bacteria into Gram-positive or Gram-negative based on the structure of their cell walls. However, mycobacteria do not stain reliably with the Gram method due to their thick, waxy cell walls, rendering it ineffective for their identification. Option C, methylene blue, is a simple stain used to observe bacterial morphology or as a counterstain in other techniques (e.g., Gram staining), but it lacks the specificity to identify mycobacteria. Option D, India ink, is used primarily to detect encapsulated organisms such as Cryptococcus neoformans by creating a negative staining effect around the capsule, and it is not suitable for mycobacteria.
The CBIC’s "Identification of Infectious Disease Processes" domain underscores the importance of accurate diagnostic methods in infection control, including the use of appropriate staining techniques to identify pathogens like mycobacteria. The acid-fast stain is specifically recommended by the CDC and WHO for the initial detection of mycobacterial infections, such as tuberculosis, in clinical specimens (CDC, Laboratory Identification of Mycobacteria, 2008). This aligns with the CBIC Practice Analysis (2022), which emphasizes the role of laboratory diagnostics in supporting infection prevention strategies.
References:
CBIC Practice Analysis, 2022.
CDC Laboratory Identification of Mycobacteria, 2008.
WHO Guidelines for the Laboratory Diagnosis of Tuberculosis, 2014.
An outbreak of Candida auris is suspected in the infection preventionist's (IP) facility. The IP's investigation must be conducted in a standard method and communication is critical. Which first step is MOST important?
Conduct environmental cultures
Plan to prevent future outbreaks
Notify facility administration
Perform analytical studies
In an outbreak investigation, the first critical step is to notify facility administration and other key stakeholders. This ensures the rapid mobilization of resources, coordination with infection control teams, and compliance with regulatory reporting requirements.
Why the Other Options Are Incorrect?
A. Conduct environmental cultures – While environmental sampling may be necessary, it is not the first step. The outbreak must first be confirmed and administration alerted.
B. Plan to prevent future outbreaks – Prevention planning happens later after the outbreak has been investigated and controlled.
D. Perform analytical studies – Data analysis occurs after case definition and initial response measures are in place.
CBIC Infection Control Reference
APIC guidelines state that the first step in an outbreak investigation is confirming the outbreak and notifying key stakeholders.
When evaluating environmental cleaning and disinfectant products as a part of the product evaluation committee, which of the following is responsible for providing information regarding clinical trials?
Infection Preventionist
Clinical representatives
Environmental Services
Manufacturer representatives
The correct answer is D, "Manufacturer representatives," as they are responsible for providing information regarding clinical trials when evaluating environmental cleaning and disinfectant products as part of the product evaluation committee. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, manufacturers are the primary source of data on the efficacy, safety, and performance of their products, including clinical trial results that demonstrate the disinfectant’s ability to reduce microbial load or prevent healthcare-associated infections (HAIs) (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.4 - Implement environmental cleaning and disinfection protocols). This information is critical for the committee to assess whether the product meets regulatory standards (e.g., EPA registration) and aligns with infection prevention goals, and it is typically supported by documentation such as peer-reviewed studies or trial data provided by the manufacturer.
Option A (Infection Preventionist) plays a key role in evaluating the product’s fit within infection control practices and may contribute expertise or conduct internal assessments, but they are not responsible for providing clinical trial data, which originates from the manufacturer. Option B (Clinical representatives) can offer insights into clinical usage and outcomes but rely on manufacturer data for trial evidence rather than generating it. Option C (Environmental Services) focuses on the practical application and cleaning processes but lacks the authority or resources to conduct or provide clinical trial information.
The reliance on manufacturer representatives aligns with CBIC’s emphasis on evidence-based decision-making in product selection, ensuring that the product evaluation committee bases its choices on robust, manufacturer-supplied clinical data (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.5 - Use data to guide infection prevention and control strategies). This approach supports the safe and effective implementation of environmental cleaning products in healthcare settings.
References: CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.5 - Use data to guide infection prevention and control strategies; Domain III: Infection Prevention and Control, Competency 3.4 - Implement environmental cleaning and disinfection protocols.
An adult with an incomplete vaccination history presents with an uncontrollable, rapid and violent cough, fever, and runny nose. Healthcare personnel should suspect
Pertussis.
Rhinovirus.
Bronchitis.
Adenovirus.
The correct answer is A, "Pertussis," as healthcare personnel should suspect this condition based on the presented symptoms and the patient’s incomplete vaccination history. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, pertussis, caused by the bacterium Bordetella pertussis, is characterized by an initial phase of mild respiratory symptoms (e.g., runny nose, low-grade fever) followed by a distinctive uncontrollable, rapid, and violent cough, often described as a "whooping" cough. This presentation is particularly concerning in adults with incomplete vaccination histories, as the pertussis vaccine’s immunity (e.g., DTaP or Tdap) wanes over time, increasing susceptibility (CBIC Practice Analysis, 2022, Domain I: Identification of Infectious Disease Processes, Competency 1.1 - Identify infectious disease processes). Pertussis is highly contagious and poses a significant risk in healthcare settings, necessitating prompt suspicion and isolation to prevent transmission.
Option B (rhinovirus) typically causes the common cold with symptoms like runny nose, sore throat, and mild cough, but it lacks the violent, paroxysmal cough characteristic of pertussis. Option C (bronchitis) may involve cough and fever, often due to viral or bacterial infection, but it is not typically associated with the rapid and violent cough pattern or linked to vaccination status in the same way as pertussis. Option D (adenovirus) can cause respiratory symptoms, including cough and fever, but it is more commonly associated with conjunctivitis or pharyngitis and does not feature the hallmark violent cough of pertussis.
The suspicion of pertussis aligns with CBIC’s emphasis on recognizing infectious disease patterns to initiate timely infection control measures, such as droplet precautions and prophylaxis for exposed individuals (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.2 - Implement measures to prevent transmission of infectious agents). Early identification is critical, especially in healthcare settings, to protect vulnerable patients and staff, and the incomplete vaccination history supports this differential diagnosis given pertussis’s vaccine-preventable nature (CDC Pink Book: Pertussis, 2021).
References: CBIC Practice Analysis, 2022, Domain I: Identification of Infectious Disease Processes, Competency 1.1 - Identify infectious disease processes; Domain III: Infection Prevention and Control, Competency 3.2 - Implement measures to prevent transmission of infectious agents. CDC Pink Book: Pertussis, 2021.
Which of the following is the correct collection technique to obtain a laboratory specimen for suspected pertussis?
Cough plate
Nares culture
Sputum culture
Nasopharyngeal culture
The gold standard specimen for diagnosing pertussis (Bordetella pertussis infection) is a nasopharyngeal culture because:
B. pertussis colonizes the nasopharynx, making it the best site for detection.
A properly collected nasopharyngeal swab or aspirate increases diagnostic sensitivity.
This method is recommended for culture, PCR, or direct fluorescent antibody testing.
Why the Other Options Are Incorrect?
A. Cough plate – Not commonly used due to low sensitivity.
B. Nares culture – The nares are not a primary site for pertussis colonization.
C. Sputum culture – B. pertussis does not commonly infect the lower respiratory tract.
CBIC Infection Control Reference
APIC confirms that nasopharyngeal culture is the preferred method for diagnosing pertussis.
The degree of infectiousness of a patient with tuberculosis correlates with
the hand-hygiene habits of the patient.
a presence of acid-fast bacilli in the blood.
a tuberculin skin test result that is greater than 20 mm
the number of organisms expelled into the air
The infectiousness of tuberculosis (TB) is directly related to the number of Mycobacterium tuberculosis organisms expelled into the air by an infected patient.
Step-by-Step Justification:
TB Transmission Mechanism:
TB spreads through airborne droplet nuclei, which remain suspended for long periods.
Factors Affecting Infectiousness:
High bacterial load in sputum: Smear-positive patients are much more infectious.
Coughing and sneezing frequency: More expelled droplets increase exposure risk.
Environmental factors: Poor ventilation increases transmission.
Why Other Options Are Incorrect:
A. Hand hygiene habits: TB is airborne, not transmitted via hands.
B. Presence of acid-fast bacilli (AFB) in blood: TB is not typically hematogenous, and blood AFB does not correlate with infectiousness.
C. Tuberculin skin test (TST) >20 mm: TST indicates prior exposure, not infectiousness.
CBIC Infection Control References:
APIC Text, "Tuberculosis Transmission and Control Measures".
An infection preventionist is reviewing practices in a facility's food preparation department. Which of the following practices should be revised?
Thawing meat at room temperature
Using a cutting board to cut vegetables
Maintaining hot food at 145° F (62.7° C) during serving
Discarding most perishable food within 72 hours
Thawing raw meat at room temperature is a major food safety violation because it allows bacteria to multiply rapidly within the temperature danger zone (40–140°F or 4.4–60°C). Meat should always be thawed in the refrigerator, under cold running water, or in a microwave if cooked immediately.
Why the Other Options Are Incorrect?
B. Using a cutting board to cut vegetables – This is safe as long as proper cleaning and sanitation procedures are followed.
C. Maintaining hot food at 145°F (62.7°C) during serving – 145°F is an acceptable minimum temperature for certain meats like beef, fish, and pork.
D. Discarding most perishable food within 72 hours – Many perishable foods, especially leftovers, should be discarded within 3 days, making this an appropriate practice.
CBIC Infection Control Reference
The APIC guidelines emphasize that raw meat should never be thawed at room temperature due to the risk of bacterial growth and foodborne illness.
An infection preventionist is evaluating a new catheter that may decrease the rate of catheter-associated urinary tract infections. Which of the following provides the BEST information to support the selection of this catheter?
Staff member preference and product availability
Product materials and vendor information
Value analysis and information provided by the manufacturer
Cost benefit analysis and safety considerations
The correct answer is D, "Cost benefit analysis and safety considerations," as this provides the best information to support the selection of a new catheter aimed at decreasing the rate of catheter-associated urinary tract infections (CAUTIs). According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, selecting medical devices like catheters for infection prevention involves a comprehensive evaluation that balances efficacy, safety, and economic impact. A cost-benefit analysis assesses the financial implications (e.g., reduced infection rates leading to lower treatment costs) against the cost of the new catheter, while safety considerations ensure the device minimizes patient risk, such as reducing biofilm formation or irritation that contributes to CAUTIs (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). This dual focus provides evidence-based data to justify the catheter’s adoption, aligning with the goal of improving patient outcomes and reducing healthcare-associated infections (HAIs).
Option A (staff member preference and product availability) is subjective and logistical rather than evidence-based, making it insufficient for a decision that impacts infection rates. Option B (product materials and vendor information) offers technical details but lacks the broader context of efficacy and cost-effectiveness needed for a comprehensive evaluation. Option C (value analysis and information provided by the manufacturer) includes a structured assessment of value, but it may be biased toward the manufacturer’s claims and lacks the independent safety and cost-benefit perspective critical for infection prevention decisions.
The emphasis on cost-benefit analysis and safety considerations reflects CBIC’s priority on using data-driven and patient-centered approaches to select interventions that enhance infection control (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.5 - Use data to guide infection prevention and control strategies). This approach ensures the catheter’s selection is supported by robust evidence, optimizing both clinical and economic outcomes in the prevention of CAUTIs.
References: CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.5 - Use data to guide infection prevention and control strategies; Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment.
Which of the following is included in an effective respiratory hygiene program in healthcare facilities?
Community educational brochures campaign
Mask availability at building entrance and reception
Separate entrance for symptomatic patients and visitors
Temperature monitoring devices at clinical unit entrance
An effective respiratory hygiene program in healthcare facilities aims to reduce the transmission of respiratory pathogens, such as influenza, COVID-19, and other droplet- or airborne infectious agents, by promoting practices that minimize the spread from infected individuals. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the importance of such programs within the "Prevention and Control of Infectious Diseases" domain, aligning with guidelines from the Centers for Disease Control and Prevention (CDC). The CDC’s "Guideline for Isolation Precautions" (2007) and its respiratory hygiene/cough etiquette recommendations outline key components, including source control, education, and environmental measures to protect patients, visitors, and healthcare workers.
Option B, "Mask availability at building entrance and reception," is a core element of an effective respiratory hygiene program. Providing masks at entry points ensures that symptomatic individuals can cover their mouth and nose, reducing the dispersal of respiratory droplets. This practice, often referred to as source control, is a primary strategy to interrupt transmission, especially in high-traffic areas like entrances and receptions. The CDC recommends that healthcare facilities offer masks or tissues and no-touch receptacles for disposal as part of respiratory hygiene, making this a practical and essential inclusion.
Option A, "Community educational brochures campaign," is a valuable adjunct to raise awareness among the public about respiratory hygiene (e.g., covering coughs, hand washing). However, it is an external strategy rather than a direct component of the facility’s internal program, which focuses on immediate action within the healthcare setting. Option C, "Separate entrance for symptomatic patients and visitors," can enhance infection control by segregating potentially infectious individuals, but it is not a universal requirement and depends on facility resources and design. The CDC suggests this as an optional measure during outbreaks, not a standard element of every respiratory hygiene program. Option D, "Temperature monitoring devices at clinical unit entrance," is a useful screening tool to identify febrile individuals, which may indicate infection. However, it is a surveillance measure rather than a core hygiene practice, and its effectiveness is limited without accompanying interventions like masking.
The CBIC Practice Analysis (2022) and CDC guidelines prioritize actionable, facility-based interventions like mask provision to mitigate transmission risks. The availability of masks at key entry points directly supports the goal of respiratory hygiene by enabling immediate source control, making Option B the most appropriate answer.
References:
CBIC Practice Analysis, 2022.
CDC Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings, 2007.
Following recent renovations on an oncology unit, three patients were identified with Aspergillus infections. The infections were thought to be facility-acquired. Appropriate environmental microbiological monitoring would be to culture the:
Air
Ice
Carpet
Aerators
The scenario describes an outbreak of Aspergillus infections among three patients on an oncology unit following recent renovations, with the infections suspected to be facility-acquired. Aspergillus is a mold commonly associated with environmental sources, particularly airborne spores, and its presence in immunocompromised patients (e.g., oncology patients) poses a significant risk. The infection preventionist must identify the appropriate environmental microbiological monitoring strategy, guided by the Certification Board of Infection Control and Epidemiology (CBIC) and CDC recommendations. Let’s evaluate each option:
A. Air: Aspergillus species are ubiquitous molds that thrive in soil, decaying vegetation, and construction dust, and they are primarily transmitted via airborne spores. Renovations can disturb these spores, leading to aerosolization and inhalation by vulnerable patients. Culturing the air using methods such as settle plates, air samplers, or high-efficiency particulate air (HEPA) filtration monitoring is a standard practice to detect Aspergillus during construction or post-renovation in healthcare settings, especially oncology units where patients are at high risk for invasive aspergillosis. This aligns with CBIC’s emphasis on environmental monitoring for airborne pathogens, making it the most appropriate choice.
B. Ice: Ice can be a source of contamination with bacteria (e.g., Pseudomonas, Legionella) or other pathogens if improperly handled or stored, but it is not a typical reservoir for Aspergillus, which is a mold requiring organic material and moisture for growth. While ice safety is important in infection control, culturing ice is irrelevant to an Aspergillus outbreak linked to renovations and is not a priority in this context.
C. Carpet: Carpets can harbor dust, mold, and other microorganisms, especially in high-traffic or poorly maintained areas. Aspergillus spores could theoretically settle in carpet during renovations, but carpets are not a primary source of airborne transmission unless disturbed (e.g., vacuuming). Culturing carpet might be a secondary step if air sampling indicates widespread contamination, but it is less direct and less commonly recommended as the initial monitoring site compared to air sampling.
D. Aerators: Aerators (e.g., faucet aerators) can harbor waterborne pathogens like Pseudomonas or Legionella due to biofilm formation, but Aspergillus is not typically associated with water systems unless there is significant organic contamination or aerosolization from water sources (e.g., cooling towers). Culturing aerators is relevant for waterborne outbreaks, not for an Aspergillus outbreak linked to renovations, making this option inappropriate.
The best answer is A, culturing the air, as Aspergillus is an airborne pathogen, and renovations are a known risk factor for spore dispersal in healthcare settings. This monitoring strategy allows the infection preventionist to confirm the source, assess the extent of contamination, and implement control measures (e.g., enhanced filtration, construction barriers) to protect patients. This is consistent with CBIC and CDC guidelines for managing fungal outbreaks in high-risk units.
References:
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain IV: Environment of Care, which recommends air sampling for Aspergillus during construction-related outbreaks.
CBIC Examination Content Outline, Domain III: Prevention and Control of Infectious Diseases, which includes environmental monitoring for facility-acquired infections.
CDC Guidelines for Environmental Infection Control in Healthcare Facilities (2022), which advocate air culturing to detect Aspergillus post-renovation in immunocompromised patient areas.
When developing an exposure control plan, the MOST important aspect in the prevention of exposure to tuberculosis is:
Placement of the patient in an airborne infection isolation room.
Identification of a potentially infectious patient.
Prompt initiation of chemotherapeutic agents.
Use of personal protective equipment.
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is an airborne disease that poses a significant risk in healthcare settings, particularly through exposure to infectious droplets. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the "Prevention and Control of Infectious Diseases" domain, which includes developing exposure control plans, aligning with the Centers for Disease Control and Prevention (CDC) "Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Healthcare Settings" (2005). The question seeks the most important aspect of an exposure control plan to prevent TB exposure, requiring a prioritization of preventive strategies.
Option B, "Identification of a potentially infectious patient," is the most important aspect. Early identification of individuals with suspected or confirmed TB (e.g., through symptom screening like persistent cough, fever, or weight loss, or diagnostic tests like chest X-rays and sputum smears) allows for timely isolation and treatment, preventing further transmission. The CDC guidelines stress that the first step in an exposure control plan is to recognize patients with signs or risk factors for infectious TB, as unrecognized cases are the primary source of healthcare worker and patient exposures. The Occupational Safety and Health Administration (OSHA) also mandates risk assessment and early detection as foundational to TB control plans.
Option A, "Placement of the patient in an airborne infection isolation room," is a critical control measure once a potentially infectious patient is identified. Airborne infection isolation rooms (AIIRs) with negative pressure ventilation reduce the spread of infectious droplets, as recommended by the CDC. However, this step depends on prior identification; placing a patient in an AIIR without knowing their infectious status is inefficient and not the initial priority. Option C, "Prompt initiation of chemotherapeutic agents," is essential for treating active TB and reducing infectiousness, typically within days of effective therapy, per CDC guidelines. However, this follows identification and diagnosis (e.g., via acid-fast bacilli smear or culture), making it a secondary action rather than the most important preventive aspect. Option D, "Use of personal protective equipment," such as N95 respirators, is a key protective measure for healthcare workers once an infectious patient is identified, as outlined by the CDC and OSHA. However, PPE is a reactive measure that mitigates exposure after identification and isolation, not the foundational step to prevent it.
The CBIC Practice Analysis (2022) and CDC guidelines prioritize early identification as the cornerstone of TB exposure prevention, enabling all subsequent interventions. Option B ensures that the exposure control plan addresses the source of transmission at its outset, making it the most important aspect.
References:
CBIC Practice Analysis, 2022.
CDC Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Healthcare Settings, 2005.
OSHA Respiratory Protection Standard, 29 CFR 1910.134.
When implementing a multimodal strategy (or bundle) for improving hand hygiene, the infection preventionist should focus on Calculator
signage for hand hygiene reminders.
cost effectiveness of hand hygiene products.
availability of gloves in the patient care area
institutional assessment of significant barriers.
When implementing a multimodal strategy (or bundle) for hand hygiene, the infection preventionist should first assess barriers to compliance before implementing solutions.
Step-by-Step Justification:
Understanding Barriers First:
Identifying barriers (e.g., lack of access to sinks, high workload, or poor compliance culture) is critical for effective intervention.
APIC Guidelines on Hand Hygiene Improvement:
Strategies must be tailored based on the institution's specific challenges.
Why Other Options Are Incorrect:
A. Signage for hand hygiene reminders:
Signage alone is insufficient without addressing systemic barriers.
B. Cost-effectiveness of hand hygiene products:
While important, cost analysis comes after identifying compliance barriers.
C. Availability of gloves in the patient care area:
Gloves do not replace hand hygiene and may lead to lower compliance.
CBIC Infection Control References:
APIC/JCR Workbook, "Hand Hygiene Compliance and Institutional Barriers".
APIC Text, "Hand Hygiene Improvement Strategies".
An infection preventionist is calculating measures of central tendency regarding duration of a surgical procedure using this data set: 2, 2, 3, 4, and 9. Which of the following statements is correct?
The median is 2.
The mode is 3.
The mean is 4.
The standard deviation is 7.
Measures of central tendency (mean, median, mode) and dispersion (standard deviation) are statistical tools used to summarize data, such as the duration of surgical procedures, which can help infection preventionists identify trends or risks for surgical site infections. The Certification Board of Infection Control and Epidemiology (CBIC) supports the use of data analysis in the "Surveillance and Epidemiologic Investigation" domain, aligning with epidemiological principles outlined by the Centers for Disease Control and Prevention (CDC). The question provides a data set of 2, 2, 3, 4, and 9, and requires determining the correct statement by calculating these measures.
Mean: The mean is the average of the data set, calculated by summing all values and dividing by the number of observations. For the data set 2, 2, 3, 4, and 9:(2 + 2 + 3 + 4 + 9) ÷ 5 = 20 ÷ 5 = 4. Thus, the mean is 4, making Option C correct.
Median: The median is the middle value when the data set is ordered. With five values (2, 2, 3, 4, 9), the middle value is the third number, which is 3. Option A states the median is 2, which is incorrect.
Mode: The mode is the most frequently occurring value. In this data set, 2 appears twice, while 3, 4, and 9 appear once each, making 2 the mode. Option B states the mode is 3, which is incorrect.
Standard Deviation: The standard deviation measures the spread of data around the mean. For a small data set like this, the calculation involves finding the variance (average of squared differences from the mean) and taking the square root. The mean is 4, so the deviations are: (2-4)² = 4, (2-4)² = 4, (3-4)² = 1, (4-4)² = 0, (9-4)² = 25. The sum of squared deviations is 4 + 4 + 1 + 0 + 25 = 34. The variance is 34 ÷ 5 = 6.8, and the standard deviation is √6.8 ≈ 2.61 (not 7). Option D states the standard deviation is 7, which is incorrect without further context (e.g., a population standard deviation with n-1 denominator would be √34 ≈ 5.83, still not 7).
The CBIC Practice Analysis (2022) and CDC guidelines encourage accurate statistical analysis to inform infection control decisions, such as assessing surgical duration as a risk factor for infections. Based on the calculations, the mean of 4 is the only correct statement among the options, confirming Option C as the answer. Note that the standard deviation of 7 might reflect a miscalculation or misinterpretation (e.g., using a different formula or data set), but with the given data, it does not hold.
References:
CBIC Practice Analysis, 2022.
CDC Principles of Epidemiology in Public Health Practice, 3rd Edition, 2012.
What question would be appropriate for an infection preventionist to ask when reviewing the discussion section of an original article?
Was the correct sample size and analysis method chosen?
Could alternative explanations account for the observed results?
Is the study question important, appropriate, and stated clearly?
Are criteria used to measure the exposure and the outcome explicit?
When reviewing the discussion section of an original article, an infection preventionist must focus on critically evaluating the interpretation of the study findings, their relevance to infection control, and their implications for practice. The discussion section typically addresses the meaning of the results, compares them to existing literature, and considers limitations or alternative interpretations. The appropriate question should align with the purpose of this section and reflect the infection preventionist's need to assess the validity and applicability of the research. Let’s analyze each option:
A. Was the correct sample size and analysis method chosen?: This question pertains to the methodology section of a research article, where the study design, sample size, and statistical methods are detailed. While these elements are critical for assessing the study's rigor, they are not the primary focus of the discussion section, which interprets results rather than re-evaluating the study design. An infection preventionist might ask this during a review of the methods section, but it is less relevant here.
B. Could alternative explanations account for the observed results?: The discussion section often explores whether the findings can be explained by factors other than the hypothesized cause, such as confounding variables, bias, or chance. This question is highly appropriate for an infection preventionist, as it encourages a critical assessment of whether the results truly support infection control interventions or if other factors (e.g., environmental conditions, patient factors) might be responsible. This aligns with CBIC's emphasis on evidence-based practice, where understanding the robustness of conclusions is key to applying research to infection prevention strategies.
C. Is the study question important, appropriate, and stated clearly?: This question relates to the introduction or background section of an article, where the research question and its significance are established. While important for overall study evaluation, it is not specific to the discussion section, which focuses on interpreting results rather than revisiting the initial question. An infection preventionist might consider this earlier in the review process, but it does not fit the context of the discussion section.
D. Are criteria used to measure the exposure and the outcome explicit?: This question is relevant to the methods section, where the definitions and measurement tools for exposures (e.g., a specific intervention) and outcomes (e.g., infection rates) are described. The discussion section may reference these criteria but focuses more on their implications rather than their clarity. This makes it less appropriate for the discussion section specifically.
The discussion section is where authors synthesize their findings, address limitations, and consider alternative explanations, making option B the most fitting. For an infection preventionist, evaluating alternative explanations is crucial to ensure that recommended practices (e.g., hand hygiene protocols or sterilization techniques) are based on solid evidence and not confounded by unaddressed variables. This critical thinking is consistent with CBIC's focus on applying research to improve infection control outcomes.
References:
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain I: Identification of Infectious Disease Processes, which emphasizes critical evaluation of research evidence.
CBIC Examination Content Outline, Domain V: Management and Communication, which includes assessing the validity of research findings for infection control decision-making.
Therapeutic antimicrobial agents should be used when
the infecting agent is unknown
the patient's illness warrants treatment prior to culture results
the patient symptoms suggest likely pathogens.
Following identification of the pathogen and sensitives.
Therapeutic antimicrobial agents should ideally be pathogen-directed to minimize resistance, side effects, and treatment failure. Once the causative pathogen and its antimicrobial susceptibilities are known, the most narrow-spectrum, effective agent should be used.
Why the Other Options Are Incorrect?
A. The infecting agent is unknown – Empiric therapy may be necessary initially, but definitive therapy should be based on pathogen identification.
B. The patient's illness warrants treatment prior to culture results – This applies to empiric therapy, but not to definitive antimicrobial selection.
C. The patient’s symptoms suggest likely pathogens – Clinical presentation guides empiric treatment, but definitive therapy should follow culture and susceptibility testing.
CBIC Infection Control Reference
APIC emphasizes the importance of selecting antimicrobials based on pathogen identification and susceptibility testing to prevent antimicrobial resistance.
In a retrospective case-control study, the initial case group is composed of persons
with the disease
without the disease.
with the risk factor under investigation
without the risk factor under investigation
In a retrospective case-control study, cases and controls are selected based on disease status. The case group is composed of individuals who have the disease (cases), while the control group consists of individuals without the disease. This design allows researchers to look back in time to assess exposure to potential risk factors.
Step-by-Step Justification:
Selection of Cases and Controls:
Cases: Individuals who already have the disease.
Controls: Individuals without the disease but similar in other aspects.
Direction of Study:
A retrospective study moves backward from the disease outcome to investigate potential causes or risk factors.
Data Collection:
Uses past medical records, interviews, and laboratory results to determine past exposures.
Common Use:
Useful for studying rare diseases since cases have already occurred, making it cost-effective compared to cohort studies.
Why Other Options Are Incorrect:
B. without the disease: (Incorrect) This describes the control group, not the case group.
C. with the risk factor under investigation: (Incorrect) Risk factors are identified after selecting cases and controls.
D. without the risk factor under investigation: (Incorrect) The study investigates whether cases had prior exposure, not whether they lacked a risk factor.
CBIC Infection Control References:
APIC Text, Chapter on Epidemiologic Study Design.
A new hospital disinfectant with a 3-minute contact time has been purchased by Environmental Services. The disinfectant will be rolled out across the patient care 3-minute contact time has been purchased by Environmental Services. The disinfectant will be rolled out across the patient care areas. They are concerned about the high cost of the disinfectant. What advice can the infection preventionist provide?
Use the new disinfectant for patient washrooms only.
Use detergents on the floors in patient rooms.
Use detergents on smooth horizontal surfaces.
Use new disinfectant for all surfaces in the patient room.
The scenario involves the introduction of a new hospital disinfectant with a 3-minute contact time, intended for use across patient care areas, but with concerns raised by Environmental Services about its high cost. The infection preventionist’s advice must balance infection control efficacy with cost management, adhering to principles outlined by the Certification Board of Infection Control and Epidemiology (CBIC) and evidence-based practices. The goal is to optimize the disinfectant’s use while ensuring a safe environment. Let’s evaluate each option:
A. Use the new disinfectant for patient washrooms only: Limiting the disinfectant to patient washrooms focuses its use on high-touch, high-risk areas where pathogens (e.g., Clostridioides difficile, norovirus) may be prevalent. However, this approach restricts the disinfectant’s application to a specific area, potentially leaving other patient care surfaces (e.g., bed rails, tables) vulnerable to contamination. While cost-saving, it does not address the broad infection control needs across all patient care areas, making it an incomplete strategy.
B. Use detergents on the floors in patient rooms: Detergents are cleaning agents that remove dirt and organic material but lack the antimicrobial properties of disinfectants. Floors in patient rooms can harbor pathogens, but they are generally considered lower-risk surfaces compared to high-touch areas (e.g., bed rails, doorknobs). Using detergents instead of the new disinfectant on floors could reduce costs but compromises infection control, as floors may still contribute to environmental transmission (e.g., via shoes or equipment). This option is not optimal given the availability of an effective disinfectant.
C. Use detergents on smooth horizontal surfaces: Smooth horizontal surfaces (e.g., tables, counters, overbed tables) are common sites for pathogen accumulation and transmission in patient rooms. Using detergents to clean these surfaces removes organic material, which is a critical first step before disinfection. If the 3-minute contact time disinfectant is reserved for high-touch or high-risk surfaces (e.g., bed rails, call buttons) where disinfection is most critical, this approach maximizes the disinfectant’s efficacy while reducing its overall use and cost. This strategy aligns with CBIC guidelines, which emphasize a two-step process (cleaning followed by disinfection) and targeted use of resources, making it a practical and cost-effective recommendation.
D. Use new disinfectant for all surfaces in the patient room: Using the disinfectant on all surfaces ensures comprehensive pathogen reduction but increases consumption and cost, which is a concern for Environmental Services. While the 3-minute contact time suggests efficiency, overusing the disinfectant on low-risk surfaces (e.g., floors, walls) may not provide proportional infection control benefits and could strain the budget. This approach does not address the cost concern and is less strategic than targeting high-risk areas.
The best advice is C, using detergents on smooth horizontal surfaces to handle routine cleaning, while reserving the new disinfectant for high-touch or high-risk areas where its antimicrobial action is most needed. This optimizes infection prevention, aligns with CBIC’s emphasis on evidence-based environmental cleaning, and addresses the cost concern by reducing unnecessary disinfectant use. The infection preventionist should also recommend a risk assessment to identify priority surfaces for disinfectant application.
References:
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain IV: Environment of Care, which advocates for targeted cleaning and disinfection based on risk.
CBIC Examination Content Outline, Domain III: Prevention and Control of Infectious Diseases, which includes cost-effective use of disinfectants.
CDC Guidelines for Environmental Infection Control in Healthcare Facilities (2022), which recommend cleaning with detergents followed by targeted disinfection.
The cleaning and disinfection process that is appropriate for a particular surgical instrument depends on
all surgical instruments are cleaned and sterilized in the same manner.
instruments contaminated with blood must be bleach cleaned first.
the device manufacturer's written instructions for use.
the policies of the sterile processing department.
The correct answer is C, "the device manufacturer's written instructions for use," as this is the factor that determines the appropriate cleaning and disinfection process for a particular surgical instrument. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, the reprocessing of surgical instruments must follow the specific instructions provided by the device manufacturer to ensure safety and efficacy. These instructions account for the instrument’s material, design, and intended use, specifying the appropriate cleaning agents, disinfection methods, sterilization techniques, and contact times to prevent damage and ensure the elimination of pathogens (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). This is also mandated by regulatory standards, such as those from the Food and Drug Administration (FDA) and the Association for the Advancement of Medical Instrumentation (AAMI), which require adherence to manufacturer guidelines to maintain device integrity and patient safety.
Option A (all surgical instruments are cleaned and sterilized in the same manner) is incorrect because different instruments have unique characteristics (e.g., materials like stainless steel vs. delicate optics), necessitating tailored reprocessing methods rather than a one-size-fits-all approach. Option B (instruments contaminated with blood must be bleach cleaned first) is a misconception; while blood contamination requires thorough cleaning, bleach is not universally appropriate and may damage certain instruments unless specified by the manufacturer. Option D (the policies of the sterile processing department) may guide internal procedures but must be based on and subordinate to the manufacturer’s instructions to ensure compliance and effectiveness.
The emphasis on manufacturer instructions aligns with CBIC’s focus on evidence-based reprocessing practices to prevent healthcare-associated infections (HAIs) and protect patients (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). Deviating from these guidelines can lead to inadequate sterilization or instrument damage, increasing infection risks.
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.3 - Ensure safe reprocessing of medical equipment, 3.5 - Evaluate the environment for infection risks. AAMI ST79:2017, Comprehensive guide to steam sterilization and sterility assurance in health care facilities.
At a facility with 10.000 employees. 5,000 are at risk for bloodbome pathogen exposure. Over the past five years, 100 of the 250 needlestick injuries involved exposure to bloodborne pathogens, and 2% of exposed employees seroconverted. How many employees became infected?
1
2
5
10
To determine the number of employees who seroconverted (became infected) after a needlestick exposure, we use the given data:
Total Needlestick Injuries: 250
Needlestick Injuries Involving Bloodborne Pathogens: 100
Seroconversion Rate: 2%
Calculation:
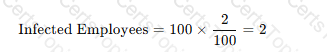 A black text with black numbers
AI-generated content may be incorrect.
A black text with black numbers
AI-generated content may be incorrect.
Why Other Options Are Incorrect:
A. 1: Incorrect calculation; 2% of 100 is 2, not 1.
C. 5: Overestimates the actual number of infections.
D. 10: Exceeds the calculated value based on given data.
CBIC Infection Control References:
APIC Text, "Occupational Exposure and Seroconversion Risks".
APIC Text, "Bloodborne Pathogens and Needlestick Injury Prevention"
Hand-hygiene audits in a long-term care facility have demonstrated consistently low levels of staff compliance. An infection preventionist is planning an education program to try to improve hand-hygiene rates. Regarding assessment of the effectiveness of the education program, which of the following is true?
A summative evaluation will accurately reflect the extent to which participants will change their hand-hygiene practices.
Repeated observations of staff will be required in order to demonstrate that the program has been effective.
A change between pre- and post-test scores correlates well with the expected change in hand-hygiene compliance.
An evaluation of the program is not required if the program is mandatory.
The correct answer is B, "Repeated observations of staff will be required in order to demonstrate that the program has been effective," as this statement is true regarding the assessment of the effectiveness of the education program. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, evaluating the impact of an education program on hand-hygiene compliance in a long-term care facility requires ongoing monitoring to assess sustained behavior change. Repeated observations provide direct evidence of staff adherence to hand-hygiene protocols over time, allowing the infection preventionist (IP) to measure the program’s effectiveness beyond initial training (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.2 - Evaluate the effectiveness of educational programs). This method aligns with the World Health Organization (WHO) and CDC recommendations for hand-hygiene improvement, which emphasize continuous auditing to ensure lasting improvements in compliance rates.
Option A (a summative evaluation will accurately reflect the extent to which participants will change their hand-hygiene practices) is incorrect because a summative evaluation, typically conducted at the end of a program, assesses overall outcomes but does not predict future behavior changes or account for long-term compliance, which is critical in this context. Option C (a change between pre- and post-test scores correlates well with the expected change in hand-hygiene compliance) is misleading; while pre- and post-tests can measure knowledge gain, they do not reliably correlate with actual practice changes, as knowledge does not always translate to behavior without observation. Option D (an evaluation of the program is not required if the program is mandatory) is false, as mandatory programs still require evaluation to verify effectiveness, especially when addressing low compliance, per CBIC and quality improvement standards.
The focus on repeated observations aligns with CBIC’s emphasis on data-driven assessment to improve infection prevention practices, ensuring that the education program leads to sustained hand-hygiene improvements and reduces healthcare-associated infections (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.4 - Evaluate the effectiveness of infection prevention and control interventions).
References: CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.4 - Evaluate the effectiveness of infection prevention and control interventions; Domain IV: Education and Research, Competency 4.2 - Evaluate the effectiveness of educational programs. WHO Guidelines on Hand Hygiene in Health Care, 2009. CDC Hand Hygiene in Healthcare Settings, 2019.
The primary source of organisms that cause surgical silo infections is the
operating room environment.
operating room personnel.
patient's endogenous flora
healthcare personnel's hands.
The primary source of organisms causing surgical site infections (SSIs) is the patient’s own endogenous flora. Bacteria from the skin, mucous membranes, or gastrointestinal tract contaminate the surgical site, leading to infection. Common pathogens include Staphylococcus aureus, coagulase-negative staphylococci, and Enterobacteriaceae.
Why the Other Options Are Incorrect?
A. Operating room environment – While environmental contamination can contribute, it is not the primary source.
B. Operating room personnel – Infection control measures (hand hygiene, gloves, masks) reduce transmission from personnel.
D. Healthcare personnel’s hands – Although hand contamination is a risk, it is secondary to the patient’s endogenous flora.
CBIC Infection Control Reference
According to APIC guidelines, the patient’s own flora is the primary source of SSIs.
During the last week in June, an emergency department log reveals numerous cases of profuse watery diarrhea in individuals 74 years of age and older. During the same time period, four immunocompromised patients were admitted with possible Cryptosporidium. Which of the following actions should the infection preventionist take FIKST?
Characterize the outbreak by person, place, and time
Increase surveillance facility wide for additional cases
Contact the laboratory to confirm stool identification results
Form a tentative hypothesis about the potential reservoir for this outbreak
When an outbreak of infectious disease is suspected, the first step is to conduct an epidemiologic investigation. This begins with characterizing the outbreak by person, place, and time to establish patterns and trends. This approach, known as descriptive epidemiology, provides critical insights into potential sources and transmission patterns.
Step-by-Step Justification:
Identify Cases and Patterns:
The infection preventionist should analyze patient demographics (person), locations of cases (place), and onset of symptoms (time). This helps in defining the outbreak scope and potential exposure sources.
Create an Epidemic Curve:
An epidemic curve helps determine whether the outbreak is a point-source or propagated event. This can indicate whether the infection is spreading person-to-person or originating from a common source.
Compare with Baseline Data:
Reviewing historical data ensures that the observed cases exceed the expected norm, confirming an outbreak.
Guide Further Investigation:
Establishing basic epidemiologic patterns guides subsequent actions, such as laboratory testing, environmental sampling, and surveillance.
Why Other Options Are Incorrect:
B. Increase surveillance facility-wide for additional cases:
While enhanced surveillance is important, it should follow the initial characterization of the outbreak. Surveillance without a defined case profile may lead to misclassification and misinterpretation.
C. Contact the laboratory to confirm stool identification results:
Confirming lab results is essential but comes after defining the outbreak's characteristics. Without an epidemiologic link, testing may yield results that are difficult to interpret.
D. Form a tentative hypothesis about the potential reservoir for this outbreak:
Hypothesis generation occurs after sufficient epidemiologic data have been collected. Jumping to conclusions without characterization may result in incorrect assumptions and ineffective control measures.
CBIC Infection Control References:
APIC Text, "Outbreak Investigations," Epidemiology, Surveillance, Performance, and Patient Safety Measures.
APIC/JCR Infection Prevention and Control Workbook, Chapter 4, Surveillance Program.
APIC Text, "Investigating Infectious Disease Outbreaks," Guidelines for Epidemic Curve Analysis.
After defining and identifying cases in a possible cluster of infections, an infection preventionist should NEXT establish:
The route of transmission.
An appropriate control group.
A hypothesis that will explain the majority of cases.
Whether observed incidence exceeds expected incidence.
When investigating a possible cluster of infections, an infection preventionist (IP) follows a structured epidemiological approach to identify the cause and implement control measures. The Certification Board of Infection Control and Epidemiology (CBIC) outlines this process within the "Surveillance and Epidemiologic Investigation" domain, which aligns with the Centers for Disease Control and Prevention (CDC) guidelines for outbreak investigation. The steps typically include defining and identifying cases, formulating a hypothesis, testing the hypothesis, and implementing control measures. The question specifies the next step after defining and identifying cases, requiring an evaluation of the logical sequence.
Option C, "A hypothesis that will explain the majority of cases," is the next critical step. After confirming a cluster through case definition and identification (e.g., by time, place, and person), the IP should develop a working hypothesis to explain the observed pattern. This hypothesis might propose a common source (e.g., contaminated equipment), a mode of transmission (e.g., airborne), or a specific population at risk. The CDC’s "Principles of Epidemiology in Public Health Practice" (3rd Edition, 2012) emphasizes that formulating a hypothesis is essential to guide further investigation, such as identifying risk factors or environmental sources. This step allows the IP to focus resources on testing the most plausible explanation before proceeding to detailed analysis or interventions.
Option A, "The route of transmission," is an important element of the investigation but typically follows hypothesis formulation. Determining the route (e.g., contact, droplet, or common vehicle) requires data collection and analysis to test the hypothesis, making it a subsequent step rather than the immediate next action. Option B, "An appropriate control group," is relevant for analytical studies (e.g., case-control studies) to compare exposed versus unexposed individuals, but this is part of hypothesis testing, which occurs after the hypothesis is established. Selecting a control group prematurely, without a hypothesis, lacks direction and efficiency. Option D, "Whether observed incidence exceeds expected incidence," is a preliminary step to define a cluster, often done during case identification using baseline data or statistical thresholds (e.g., exceeding the mean plus two standard deviations). Since the question assumes cases are already defined and identified, this step is complete, and the focus shifts to hypothesis development.
The CBIC Practice Analysis (2022) and CDC guidelines prioritize hypothesis formulation as the logical next step after case identification, enabling a targeted investigation. This approach ensures that the IP can efficiently address the cluster’s cause, making Option C the correct answer.
References:
CBIC Practice Analysis, 2022.
CDC Principles of Epidemiology in Public Health Practice, 3rd Edition, 2012.
A nurse exposed to pertussis develops a mild cough 14 days later. What is the recommended action?
Continue working with a surgical mask.
Exclude from patient care until five days after starting antibiotics.
Initiate post-exposure prophylaxis only if symptoms worsen.
Conduct serologic testing before deciding on work restrictions.
The CDC recommends exclusion of healthcare workers with pertussis until completing at least five days of antibiotic therapy.
CBIC Infection Control References:
APIC-JCR Workbook, "Occupational Health Considerations," Chapter 10
In the current year, cases of tuberculosis (TB) among foreign-born persons accounted for the majority of new TB cases in the United States. The number of states with greater than 50% of cases among foreign-born persons increased from four cases ten years ago to 22 cases in the current year. This information can BEST be used to
heighten awareness among Emergency Department staff.
inform staff who are foreign-born.
educate patients and visitors.
review the TB exposure control plan.
1 and 2 only.
1 and 4 only.
2 and 3 only.
3 and 4 only.
The correct answer is B, "1 and 4 only," indicating that the information can best be used to heighten awareness among Emergency Department (ED) staff and review the TB exposure control plan. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, tuberculosis (TB) remains a significant public health concern, particularly with the increasing proportion of cases among foreign-born persons in the United States. The data showing a rise from four to 22 states with over 50% of TB cases among foreign-born individuals highlights an evolving epidemiological trend that warrants targeted infection prevention strategies (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.1 - Conduct surveillance for healthcare-associated infections and epidemiologically significant organisms).
Heightening awareness among ED staff (option 1) is critical because the ED is often the first point of contact for patients with undiagnosed or active TB, especially those from high-prevalence regions. Increased awareness can improve early identification, isolation, and reporting of potential cases. Reviewing the TB exposure control plan (option 4) is equally important, as it allows the infection preventionist to assess and update protocols—such as ventilation, personal protective equipment (PPE) use, and screening processes—to address the heightened risk posed by the growing number of cases among foreign-born individuals (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.2 - Implement measures to prevent transmission of infectious agents).
Option 2 (inform staff who are foreign-born) is not the best use of this data, as the information pertains to patient demographics rather than staff risk, and targeting staff based on their origin could be inappropriate without specific exposure evidence. Option 3 (educate patients and visitors) is a general education strategy but less directly actionable with this specific epidemiological data, which is more relevant to healthcare worker preparedness and facility protocols. Combining options 1 and 4 aligns with CBIC’s emphasis on using surveillance data to guide prevention and control measures, ensuring a proactive response to the increased TB burden (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.5 - Use data to guide infection prevention and control strategies).
References: CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competencies 2.1 - Conduct surveillance for healthcare-associated infections and epidemiologically significant organisms, 2.5 - Use data to guide infection prevention and control strategies; Domain III: Infection Prevention and Control, Competency 3.2 - Implement measures to prevent transmission of infectious agents.
The infection preventionist (IP) is assisting pharmacists in investigating medication contamination at the hospital’s compounding pharmacy. As part of the medication recall process, the IP should:
Have laboratory culture all medication.
Inspect for safe injection practices.
Identify the potential source of contamination.
Inform all discharged patients of potential medication contamination.
The scenario involves an infection preventionist (IP) assisting pharmacists in addressing medication contamination at the hospital’s compounding pharmacy, with a focus on the medication recall process. The IP’s role is to apply infection control expertise to mitigate risks, guided by the Certification Board of Infection Control and Epidemiology (CBIC) principles and best practices. The recall process requires a systematic approach to identify, contain, and resolve the issue, and the “first” or most critical step must be determined. Let’s evaluate each option:
A. Have laboratory culture all medication: Culturing all medication to confirm contamination is a valuable step to identify affected batches and guide the recall. However, this is a resource-intensive process that depends on first understanding the scope and source of the problem. Without identifying the potential source of contamination, culturing all medication could be inefficient and delay the recall. This step is important but secondary to initial investigation.
B. Inspect for safe injection practices: Inspecting for safe injection practices (e.g., single-use vials, proper hand hygiene, sterile technique) is a critical infection control measure, especially in compounding pharmacies where contamination often arises from procedural errors (e.g., reuse of syringes, improper cleaning). While this is a proactive step to prevent future contamination, it addresses ongoing practices rather than the immediate recall process for the current contamination event. It is a complementary action but not the first priority.
C. Identify the potential source of contamination: Identifying the potential source of contamination is the foundational step in the recall process. This involves investigating the compounding environment (e.g., water quality, equipment, personnel practices), raw materials, and production processes to pinpoint where the contamination occurred (e.g., bacterial ingress, cross-contamination). The CBIC emphasizes root cause analysis as a key infection prevention strategy, enabling targeted recalls, corrective actions, and prevention of recurrence. This step is essential before culturing, inspecting, or notifying patients, making it the IP’s primary responsibility in this context.
D. Inform all discharged patients of potential medication contamination: Notifying patients is a critical step to ensure public safety and allow for medical follow-up if they received contaminated medication. However, this action requires prior identification of the contaminated batches and their distribution, which depends on determining the source and confirming the extent of the issue. Premature notification without evidence could cause unnecessary alarm and is not the first step in the recall process.
The best answer is C, as identifying the potential source of contamination is the initial and most critical step in the medication recall process. This allows the IP to collaborate with pharmacists to trace the contamination, define the affected products, and guide subsequent actions (e.g., culturing, inspections, notifications). This aligns with CBIC’s focus on systematic investigation and risk mitigation in healthcare-associated infection events.
References:
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain III: Prevention and Control of Infectious Diseases, which includes identifying sources of contamination in healthcare settings.
CBIC Examination Content Outline, Domain V: Management and Communication, which emphasizes root cause analysis during outbreak investigations.
CDC Guidelines for Safe Medication Compounding (2022), which recommend identifying contamination sources as the first step in a recall process.
An infection preventionist, Cancer Committee, and Intravenous Therapy Department are studying the incidence of infections in patients with triple lumen catheters. Which of the following is essential to the quality improvement process?
Establish subjective criteria for outcome measurement.
Recommendations for intervention must be approved by the governing board.
Study criteria must be approved monthly by the Cancer Committee.
A monitoring system must be in place following implementation of interventions.
The correct answer is D, "A monitoring system must be in place following implementation of interventions," as this is essential to the quality improvement (QI) process. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, a key component of any QI initiative, such as studying the incidence of infections in patients with triple lumen catheters, is the continuous evaluation of interventions to assess their effectiveness and ensure sustained improvement. A monitoring system allows the infection preventionist (IP), Cancer Committee, and Intravenous Therapy Department to track infection rates, identify trends, and make data-driven adjustments to infection control practices post-intervention (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.4 - Evaluate the effectiveness of infection prevention and control interventions). This step is critical to validate the success of implemented strategies, such as catheter care protocols, and to prevent healthcare-associated infections (HAIs).
Option A (establish subjective criteria for outcome measurement) is not ideal because QI processes rely on objective, measurable outcomes (e.g., infection rates per 1,000 catheter days) rather than subjective criteria to ensure reliability and reproducibility. Option B (recommendations for intervention must be approved by the governing board) is an important step for institutional support and resource allocation, but it is a preparatory action rather than an essential component of the ongoing QI process itself. Option C (study criteria must be approved monthly by the Cancer Committee) suggests an unnecessary administrative burden; while initial approval of study criteria is important, monthly re-approval is not a standard QI requirement unless mandated by specific policies, and it does not directly contribute to the improvement process.
The emphasis on a monitoring system aligns with CBIC’s focus on using surveillance data to guide and refine infection prevention efforts, ensuring that interventions for triple lumen catheter-related infections are effective and adaptable (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.5 - Use data to guide infection prevention and control strategies). This approach supports a cycle of continuous improvement, which is foundational to reducing catheter-associated bloodstream infections (CABSI) in healthcare settings.
References: CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competencies 2.4 - Evaluate the effectiveness of infection prevention and control interventions, 2.5 - Use data to guide infection prevention and control strategies.
An 84-year-old male with a gangrenous foot is admitted to the hospital from an extended-care facility (ECF). The ECF is notified that the wound grew Enterococcus faecium with the following antibiotic sensitivity results:
ampicillin – R
vancomycin – R
penicillin – R
linezolid – S
This is the fourth Enterococcus species cultured from residents within the same ECF wing in the past month. The other cultures were from two urine specimens and a draining wound. The Infection Preventionist (IP) should immediately:
Notify the medical director of the outbreak.
Compare the four culture reports and sensitivity patterns.
Conduct surveillance cultures for this organism in all residents.
Notify the nursing administrator to close the wing to new admissions.
The scenario describes a potential outbreak of multidrug-resistant Enterococcus faecium in an extended-care facility (ECF) wing, indicated by four positive cultures (including the current case and three prior cases from urine and a draining wound) within a month. The organism exhibits resistance to ampicillin, vancomycin, and penicillin, but sensitivity to linezolid, suggesting a possible vancomycin-resistant Enterococcus (VRE) strain, which is a significant concern in healthcare settings. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the importance of rapid outbreak detection and response in the "Surveillance and Epidemiologic Investigation" domain, aligning with Centers for Disease Control and Prevention (CDC) guidelines for managing multidrug-resistant organisms (MDROs).
Option A, "Notify the medical director of the outbreak," is the most immediate and critical action. Identifying an outbreak—defined by the CDC as two or more cases of a similar illness linked by time and place—requires prompt notification to the facility’s leadership (e.g., medical director) to initiate a coordinated response. The presence of four Enterococcus cases, including a multidrug-resistant strain, within a single ECF wing over a month suggests a potential cluster, necessitating urgent action to assess the scope, implement control measures, and allocate resources. The CDC’s "Management of Multidrug-Resistant Organisms in Healthcare Settings" (2006) recommends immediate reporting to facility leadership as the first step to activate an outbreak investigation team, making this the priority.
Option B, "Compare the four culture reports and sensitivity patterns," is an important subsequent step in outbreak investigation. Analyzing the antibiotic susceptibility profiles and culture sources can confirm whether the cases are epidemiologically linked (e.g., clonal spread of VRE) and guide treatment and control strategies. However, this is a detailed analysis that follows initial notification and should not delay alerting the medical director. Option C, "Conduct surveillance cultures for this organism in all residents," is a proactive measure to determine the prevalence of Enterococcus faecium, especially VRE, within the wing. The CDC recommends targeted surveillance during outbreaks, but this requires prior authorization and planning by the outbreak team, making it a secondary action after notification. Option D, "Notify the nursing administrator to close the wing to new admissions," may be a control measure to prevent further spread, as suggested by the CDC for MDRO outbreaks. However, closing a unit is a significant decision that should be guided by the medical director and infection control team after assessing the situation, not an immediate independent action by the IP.
The CBIC Practice Analysis (2022) and CDC guidelines prioritize rapid communication with leadership to initiate a structured outbreak response, including resource allocation and policy adjustments. Given the multidrug-resistant nature and cluster pattern, notifying the medical director (Option A) is the most immediate and appropriate action to ensure a comprehensive response.
References:
CBIC Practice Analysis, 2022.
CDC Management of Multidrug-Resistant Organisms in Healthcare Settings, 2006.
The Infection Prevention and Control Committee is concerned about an outbreak of Serratia marcescens in the intensive care unit. If an environmental source is suspected, the BEST method to validate this suspicion is to
apply fluorescent gel.
use ATP system.
obtain surface cultures.
perform direct practice observation.
The correct answer is C, "obtain surface cultures," as this is the best method to validate the suspicion of an environmental source for an outbreak of Serratia marcescens in the intensive care unit (ICU). According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, Serratia marcescens is an opportunistic gram-negative bacterium commonly associated with healthcare-associated infections (HAIs), often linked to contaminated water, medical equipment, or environmental surfaces in ICUs. Obtaining surface cultures allows the infection preventionist (IP) to directly test environmental samples (e.g., from sinks, ventilators, or countertops) for the presence of Serratia marcescens, providing microbiological evidence to confirm or rule out an environmental source (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.2 - Analyze surveillance data). This method is considered the gold standard for outbreak investigations when an environmental reservoir is suspected, as it offers specific pathogen identification and supports targeted interventions.
Option A (apply fluorescent gel) is a technique used to assess cleaning efficacy by highlighting areas missed during disinfection, but it does not directly identify the presence of Serratia marcescens or confirm an environmental source. Option B (use ATP system) measures adenosine triphosphate (ATP) to evaluate surface cleanliness and organic residue, which can indicate poor cleaning practices, but it is not specific to detecting Serratia marcescens and lacks the diagnostic precision of cultures. Option D (perform direct practice observation) is valuable for assessing staff adherence to infection control protocols, but it addresses human factors rather than directly validating an environmental source, making it less relevant as the initial step in this context.
The focus on obtaining surface cultures aligns with CBIC’s emphasis on using evidence-based methods to investigate and control HAIs, enabling the IP to collaborate with the committee to pinpoint the source and implement corrective measures (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.3 - Identify risk factors for healthcare-associated infections). This approach is supported by CDC guidelines for outbreak investigations, which prioritize microbiological sampling to guide environmental control strategies (CDC Guidelines for Environmental Infection Control in Healthcare Facilities, 2019).
References: CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competencies 2.2 - Analyze surveillance data, 2.3 - Identify risk factors for healthcare-associated infections. CDC Guidelines for Environmental Infection Control in Healthcare Facilities, 2019.
A healthcare personnel has an acute group A streptococcal throat infection. What is the earliest recommended time that this person may return to work after receiving appropriate antibiotic therapy?
8 hours
24 hours
48 hours
72 hours
The correct answer is B, "24 hours," as this is the earliest recommended time that a healthcare personnel with an acute group A streptococcal throat infection may return to work after receiving appropriate antibiotic therapy. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, which align with recommendations from the Centers for Disease Control and Prevention (CDC), healthcare workers with group A Streptococcus (GAS) infections, such as streptococcal pharyngitis, should be treated with antibiotics (e.g., penicillin or a suitable alternative) to eradicate the infection and reduce transmission risk. The CDC and Occupational Safety and Health Administration (OSHA) guidelines specify that healthcare personnel can return to work after at least 24 hours of effective antibiotic therapy, provided they are afebrile and symptoms are improving, as this period is sufficient to significantly reduce the bacterial load and contagiousness (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.2 - Implement measures to prevent transmission of infectious agents).
Option A (8 hours) is too short a duration to ensure the infection is adequately controlled and the individual is no longer contagious. Option C (48 hours) and Option D (72 hours) are longer periods that may apply in some cases (e.g., if symptoms persist or in outbreak settings), but they exceed the minimum recommended time based on current evidence. The 24-hour threshold is supported by studies showing that GAS shedding decreases substantially within this timeframe with appropriate antibiotic treatment, minimizing the risk to patients and colleagues (CDC Guidelines for Infection Control in Healthcare Personnel, 2019).
The infection preventionist’s role includes enforcing return-to-work policies to prevent healthcare-associated infections (HAIs), aligning with CBIC’s emphasis on timely and evidence-based interventions to control infectious disease transmission in healthcare settings (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.1 - Collaborate with organizational leaders). Compliance with this recommendation also supports occupational health protocols to balance staff safety and patient care.
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.1 - Collaborate with organizational leaders, 3.2 - Implement measures to prevent transmission of infectious agents. CDC Guidelines for Infection Control in Healthcare Personnel, 2019.
A patient with a non-crusted rash has boon diagnosed with Sarcoptes scabiei. The patient is treated with 5% permethrin and precautions are started. The precautions can be stopped
when the treatment cream is applied
when the bed linen is changed
24 hours after effective treatment
24 hours after the second treatment
For Sarcoptes scabiei (scabies), Contact Precautions should remain in place until 24 hours after effective treatment has been completed. The first-line treatment is 5% permethrin cream, which is applied to the entire body and left on for 8–14 hours before being washed off.
Why the Other Options Are Incorrect?
A. When the treatment cream is applied – The mite is still present and infectious until treatment has fully taken effect.
B. When the bed linen is changed – While changing linens is necessary, it does not indicate that the infestation has cleared.
D. 24 hours after the second treatment – Most cases require only one treatment with permethrin, though severe cases may need a second dose after a week.
CBIC Infection Control Reference
According to APIC guidelines, Contact Precautions can be discontinued 24 hours after effective treatment has been administered.
An infection preventionist is preparing a report about an outbreak of scabies in a long-term care facility. How would this information be displayed in an epidemic curve?
List case names, room numbers, and date the infestation was identified using a logarithmic scale.
List case medical record numbers and the number of days in the facility to date of onset, showing data in a scatter plot.
Prepare a bar graph with no patient identifiers showing the number of cases over a specific period of time.
Prepare a scatter plot by patient location showing case prevalence over a specific period of time.
An epidemic curve, commonly used in infection prevention and control to visualize the progression of an outbreak, is a graphical representation of the number of cases over time. According to the principles outlined by the Certification Board of Infection Control and Epidemiology (CBIC), an epidemic curve is most effectively displayed using a bar graph or histogram that tracks the number of new cases by date or time interval (e.g., daily, weekly) without revealing patient identifiers, ensuring compliance with privacy regulations such as HIPAA. Option C aligns with this standard practice, as it specifies preparing a bar graph with no patient identifiers, focusing solely on the number of cases over a specific period. This allows infection preventionists to identify patterns, such as the peak of the outbreak or potential sources of transmission, while maintaining confidentiality.
Option A is incorrect because listing case names and room numbers with a logarithmic scale violates patient privacy and is not a standard method for constructing an epidemic curve. Logarithmic scales are typically used for data with a wide range of values, but they are not the preferred format for epidemic curves, which prioritize clarity over time. Option B is also incorrect, as using medical record numbers and scatter plots to show days in the facility to onset does not align with the definition of an epidemic curve, which focuses on case counts over time rather than individual patient timelines or scatter plot formats. Option D is inappropriate because a scatter plot by patient location emphasizes spatial distribution rather than the temporal progression central to an epidemic curve. While location data can be useful in outbreak investigations, it is typically analyzed separately from the epidemic curve.
The CBIC emphasizes the importance of epidemic curves in the "Identification of Infectious Disease Processes" domain, where infection preventionists use such tools to monitor and control outbreaks (CBIC Practice Analysis, 2022). Specifically, the use of anonymized data in graphical formats is a best practice to protect patient information while providing actionable insights, as detailed in the CBIC Infection Prevention and Control (IPC) guidelines.
References:
CBIC Practice Analysis, 2022.
CBIC Infection Prevention and Control Guidelines (IPC), Section on Outbreak Investigation and Epidemic Curve Construction.
Which of the following is an essential element of practice when sending biohazardous samples from one location to another?
Ship using triple-containment packaging
Electronically log and send via overnight delivery
Transport by an authorized biohazard transporter
Store in a cooler that is labeled as a health hazard
The safe transport of biohazardous samples, such as infectious agents, clinical specimens, or diagnostic materials, is a critical aspect of infection prevention and control to prevent exposure and environmental contamination. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes adherence to regulatory and safety standards in the "Prevention and Control of Infectious Diseases" domain, which includes proper handling and shipping of biohazardous materials. The primary guideline governing this practice is the U.S. Department of Transportation (DOT) Hazardous Materials Regulations (HMR) and the International Air Transport Association (IATA) Dangerous Goods Regulations, which align with global biosafety standards.
Option A, "Ship using triple-containment packaging," is the essential element of practice. Triple-containment packaging involves three layers: a primary watertight container holding the sample, a secondary leak-proof container with absorbent material, and an outer rigid packaging (e.g., a box) that meets shipping regulations. This system ensures that biohazardous materials remain secure during transport, preventing leaks or breaches that could expose handlers or the public. The CDC and WHO endorse this method as a fundamental requirement for shipping Category A (high-risk) and Category B (moderate-risk) infectious substances, making it the cornerstone of safe transport practice.
Option B, "Electronically log and send via overnight delivery," is a useful administrative and logistical step to track shipments and ensure timely delivery, but it is not the essential element. While documentation and rapid delivery are important for maintaining chain of custody and sample integrity, they are secondary to the physical containment provided by triple packaging. Option C, "Transport by an authorized biohazard transporter," is a necessary step to comply with regulations, as only trained and certified transporters can handle biohazardous materials. However, this is contingent on proper packaging; without triple containment, transport authorization alone is insufficient. Option D, "Store in a cooler that is labeled as a health hazard," may be part of preparation (e.g., maintaining sample temperature), but labeling alone does not address the containment or transport safety required during shipment. Coolers are often used, but the focus on labeling as a health hazard is incomplete without the triple-containment structure.
The CBIC Practice Analysis (2022) supports compliance with federal and international shipping regulations, which prioritize triple-containment packaging as the foundational practice to mitigate risks. The CDC’s Biosafety in Microbiological and Biomedical Laboratories (BMBL, 6th Edition, 2020) and IATA guidelines further specify that triple packaging is mandatory for all biohazardous shipments, reinforcing Option A as the correct answer.
References:
CBIC Practice Analysis, 2022.
CDC Biosafety in Microbiological and Biomedical Laboratories (BMBL), 6th Edition, 2020.
U.S. DOT Hazardous Materials Regulations (49 CFR Parts 171-180).
IATA Dangerous Goods Regulations, 2023.
Healthcare workers are MOST likely to benefit from infection prevention education if the Infection Preventionist (IP)
brings in speakers who are recognized experts.
plans the educational program well ahead of time.
audits practices and identifies deficiencies.
involves the staff in determining the content.
The correct answer is D, "involves the staff in determining the content," as this approach is most likely to benefit healthcare workers from infection prevention education. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, effective education programs are tailored to the specific needs and contexts of the learners. Involving staff in determining the content ensures that the educational material addresses their real-world challenges, knowledge gaps, and interests, thereby increasing engagement, relevance, and application of the learned principles (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.1 - Develop and implement educational programs). This participatory approach fosters ownership and accountability among healthcare workers, enhancing the likelihood that they will adopt and sustain infection prevention practices.
Option A (brings in speakers who are recognized experts) can enhance credibility and provide high-quality information, but it does not guarantee that the content will meet the specific needs of the staff unless their input is considered. Option B (plans the educational program well ahead of time) is important for logistical success and preparedness, but without staff involvement, the program may lack relevance or fail to address immediate concerns. Option C (audits practices and identifies deficiencies) is a valuable step in identifying areas for improvement, but it is a diagnostic process rather than a direct educational strategy; education based solely on audits might not engage staff effectively if their input is not sought.
The focus on involving staff aligns with CBIC’s emphasis on adult learning principles, which highlight the importance of learner-centered education. By involving staff, the IP adheres to best practices for adult education, ensuring that the program is practical and tailored, ultimately leading to better outcomes in infection prevention (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.2 - Evaluate the effectiveness of educational programs). This approach also supports a collaborative culture, which is critical for sustaining infection control efforts in healthcare settings.
References: CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competencies 4.1 - Develop and implement educational programs, 4.2 - Evaluate the effectiveness of educational programs.
Immediate use steam sterilization is NOT recommended for implantable items requiring immediate use because
the high temperature may damage the items.
chemical indicators may not be accurate at high temperatures.
results of biologic indicators are unavailable prior to use of the item.
the length of time is inadequate for the steam to penetrate the pack.
The correct answer is C, "results of biologic indicators are unavailable prior to use of the item," as this is the primary reason immediate use steam sterilization (IUSS) is not recommended for implantable items requiring immediate use. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, IUSS is a process used for sterilizing items needed urgently when no other sterile options are available, typically involving a shortened cycle (e.g., flash sterilization). However, for implantable items—such as orthopedic hardware or prosthetic devices—ensuring absolute sterility is critical due to the risk of deep infection. Biologic indicators (BIs), which contain highly resistant spores to verify sterilization efficacy, require incubation (typically 24-48 hours) to confirm the kill, but IUSS does not allow time for BI results to be available before the item is used (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). This lack of immediate verification poses a significant infection risk, making IUSS inappropriate for implants, as per AAMI ST79 standards.
Option A (the high temperature may damage the items) is a consideration for some heat-sensitive materials, but modern IUSS cycles are designed to minimize damage, and this is not the primary reason for the restriction on implants. Option B (chemical indicators may not be accurate at high temperatures) is incorrect, as chemical indicators (e.g., color-changing strips) are reliable at high temperatures and serve as an immediate check, though they are not a substitute for BIs. Option D (the length of time is inadequate for the steam to penetrate the pack) is not the main issue, as IUSS cycles are optimized for penetration, though the shortened time may be a secondary concern; the unavailability of BI results remains the decisive factor.
The focus on biologic indicator results aligns with CBIC’s emphasis on ensuring the safety and sterility of reprocessed medical devices, particularly for high-risk implantable items (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). This recommendation is supported by AAMI and CDC guidelines, which prioritize BI confirmation for implants to prevent healthcare-associated infections (AAMI ST79:2017, CDC Sterilization Guidelines, 2019).
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.3 - Ensure safe reprocessing of medical equipment, 3.5 - Evaluate the environment for infection risks. AAMI ST79:2017, Comprehensive guide to steam sterilization and sterility assurance in health care facilities. CDC Guidelines for Disinfection and Sterilization in Healthcare Facilities, 2019.
Which water type is suitable for drinking yet may still be a risk for disease transmission?
Purified water
Grey water
Potable water
Distilled water
To determine which water type is suitable for drinking yet may still pose a risk for disease transmission, we need to evaluate each option based on its definition, treatment process, and potential for contamination, aligning with infection control principles as outlined by the Certification Board of Infection Control and Epidemiology (CBIC).
A. Purified water: Purified water undergoes a rigorous treatment process (e.g., reverse osmosis, distillation, or deionization) to remove impurities, contaminants, and microorganisms. This results in water that is generally safe for drinking and has a very low risk of disease transmission when properly handled and stored. However, if the purification process is compromised or if contamination occurs post-purification (e.g., due to improper storage or distribution), there could be a theoretical risk. Nonetheless, purified water is not typically considered a primary source of disease transmission under standard conditions.
B. Grey water: Grey water refers to wastewater generated from domestic activities such as washing dishes, laundry, or bathing, which may contain soap, food particles, and small amounts of organic matter. It is not suitable for drinking due to its potential contamination with pathogens (e.g., bacteria, viruses) and chemicals. Grey water is explicitly excluded from potable water standards and poses a significant risk for disease transmission, making it an unsuitable choice for this question.
C. Potable water: Potable water is water that meets regulatory standards for human consumption, as defined by organizations like the World Health Organization (WHO) or the U.S. Environmental Protection Agency (EPA). It is treated to remove harmful pathogens and contaminants, making it safe for drinking under normal circumstances. However, despite treatment, potable water can still pose a risk for disease transmission if the distribution system is contaminated (e.g., through biofilms, cross-connections, or inadequate maintenance of pipes). Outbreaks of waterborne diseases like Legionnaires' disease or gastrointestinal infections have been linked to potable water systems, especially in healthcare settings. This makes potable water the best answer, as it is suitable for drinking yet can still carry a risk under certain conditions.
D. Distilled water: Distilled water is produced by boiling water and condensing the steam, which removes most impurities, minerals, and microorganisms. It is highly pure and safe for drinking, often used in medical and laboratory settings. Similar to purified water, the risk of disease transmission is extremely low unless contamination occurs after distillation due to improper handling or storage. Like purified water, it is not typically associated with disease transmission risks in standard use.
The key to this question lies in identifying a water type that is both suitable for drinking and has a documented potential for disease transmission. Potable water fits this criterion because, while it is intended for consumption and meets safety standards, it can still be a vector for disease if the water supply or distribution system is compromised. This is particularly relevant in infection control, where maintaining water safety in healthcare facilities is a critical concern addressed by CBIC guidelines.
References:
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain III: Prevention and Control of Infectious Diseases, which highlights the importance of water safety and the risks of contamination in potable water systems.
CBIC Examination Content Outline, Domain IV: Environment of Care, which includes managing waterborne pathogens (e.g., Legionella) in potable water supplies.
A 17-year-old presents to the Emergency Department with fever, stiff neck, and vomiting. A lumbar puncture is done. The Gram stain shows Gram negative diplocooci. Presumptive identification of the organism is
Haemophilus influenzae
Neisseria meningitidis
Listeria monocytogenes
Streptococcus pneumoniae
The Gram stain showing Gram-negative diplococci in cerebrospinal fluid (CSF) is characteristic of Neisseria meningitidis, a leading cause of bacterial meningitis in adolescents and young adults.
Step-by-Step Justification:
Gram Stain Interpretation:
Gram-negative diplococci in CSF strongly suggest Neisseria meningitidis.
Classic Symptoms of Meningitis:
Fever, stiff neck, and vomiting are hallmark signs of meningococcal meningitis.
Neisseria meningitidis vs. Other Bacteria:
Haemophilus influenzae (Option A) → Gram-negative coccobacilli.
Listeria monocytogenes (Option C) → Gram-positive rods.
Streptococcus pneumoniae (Option D) → Gram-positive diplococci.
CBIC Infection Control References:
APIC Ready Reference for Microbes, "Neisseria meningitidis and Meningitis".
A patient has a draining sinus at the site of a left total hip arthroplasty. A culture from the sinus tract reveals four organisms. Which of the following specimens is optimal for identifying the eliologic agent?
Blood
Wound drainage
Joint aspirate
Sinus tract tissue
The optimal specimen for identifying the etiologic agent in a prosthetic joint infection (PJI) is a joint aspirate (synovial fluid). This is because:
It provides direct access to the infected site without contamination from external sources.
It allows for accurate microbiologic culture, Gram stain, and leukocyte count analysis.
Why the Other Options Are Incorrect?
A. Blood – Blood cultures may help detect hematogenous spread but are not the best sample for identifying localized prosthetic joint infections.
B. Wound drainage – Wound cultures often contain contaminants from surrounding skin flora and do not accurately reflect joint space infection.
D. Sinus tract tissue – Cultures from sinus tracts often represent colonization rather than the primary infecting organism.
CBIC Infection Control Reference
APIC guidelines confirm that joint aspirate is the most reliable specimen for diagnosing prosthetic joint infections.
The annual report for Infection Prevention shows a dramatic decrease in urinary catheter days, a decrease in the catheter utilization ratio, and a slight decrease in the number of catheter-associated urinary tract infections (CAUTIs). The report does not show an increase in the overall rate of CAUTI. How would the infection preventionist explain this to the administration?
The rate is incorrect and needs to be recalculated.
The rate may be higher if the denominator is very small.
The rate is not affected by the number of catheter days.
Decreasing catheter days will not have an effect on decreasing CAUTI.
The correct answer is B, "The rate may be higher if the denominator is very small," as this provides the most plausible explanation for the observed data in the annual report. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, the CAUTI rate is calculated as the number of CAUTIs per 1,000 catheter days, where catheter days serve as the denominator. The report indicates a dramatic decrease in urinary catheter days and a slight decrease in the number of CAUTIs, yet the overall CAUTI rate has not increased. This discrepancy can occur if the denominator (catheter days) becomes very small, which can inflate or destabilize the rate, potentially masking an actual increase in the infection risk per catheter day (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.2 - Analyze surveillance data). A smaller denominator amplifies the impact of even a slight change in the number of infections, suggesting that the rate may be higher than expected or less reliable, necessitating further investigation.
Option A (the rate is incorrect and needs to be recalculated) assumes an error in the calculation without evidence, which is less specific than the denominator effect explanation. Option C (the rate is not affected by the number of catheter days) is incorrect because the CAUTI rate is directly influenced by the number of catheter days as the denominator; a decrease in catheter days should typically lower the rate if infections decrease proportionally, but the lack of an increase here suggests a calculation or interpretation issue. Option D (decreasing catheter days will not have an effect on decreasing CAUTI) contradicts evidence-based practice, as reducing catheter days is a proven strategy to lower CAUTI incidence, though the rate’s stability here indicates a potential statistical artifact.
The explanation focusing on the denominator aligns with CBIC’s emphasis on accurate surveillance and data analysis to guide infection prevention strategies, allowing the infection preventionist to advise administration on the need to review data trends or adjust monitoring methods (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.5 - Use data to guide infection prevention and control strategies). This insight can prompt a deeper analysis to ensure the CAUTI rate reflects true infection risk.
References: CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competencies 2.2 - Analyze surveillance data, 2.5 - Use data to guide infection prevention and control strategies.
Which of the following descriptions accurately describes a single-use medical device?
A device which can be used on a single patient
A device that is sterilized and can be used again on the same patient
A device used on a patient and reprocessed prior to being used again
A device used one time on a patient during a procedure and then discarded
The correct answer is D, "A device used one time on a patient during a procedure and then discarded," as this accurately describes a single-use medical device. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, a single-use device (SUD), also known as a disposable device, is labeled by the manufacturer for one-time use on a patient and is intended to be discarded afterward to prevent cross-contamination and ensure patient safety. This definition is consistent with regulations from the Food and Drug Administration (FDA), which designate SUDs as devices that should not be reprocessed or reused due to risks of infection, material degradation, or failure to restore sterility (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). Examples include certain syringes, catheters, and gloves, which are designed for single use to eliminate the risk of healthcare-associated infections (HAIs).
Option A (a device which can be used on a single patient) is too vague and could apply to both single-use and reusable devices, as reusable devices are also often used on a single patient per procedure before reprocessing. Option B (a device that is sterilized and can be used again on the same patient) describes a reusable device, not a single-use device, as sterilization and reuse are not permitted for SUDs. Option C (a device used on a patient and reprocessed prior to being used again) refers to a reusable device that undergoes reprocessing (e.g., sterilization), which is explicitly prohibited for SUDs under manufacturer and regulatory guidelines.
The focus on discarding after one use aligns with CBIC’s emphasis on preventing infection through adherence to device labeling and safe reprocessing practices, ensuring that healthcare facilities avoid the risks associated with improper reuse of SUDs (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). This practice is critical to maintaining a sterile and safe healthcare environment.
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.3 - Ensure safe reprocessing of medical equipment, 3.5 - Evaluate the environment for infection risks. FDA Guidance on Reprocessing of Single-Use Devices, 2016.
Copyright © 2021-2025 CertsTopics. All Rights Reserved

